The New Way to Success Advance Progress
The clinical studies are becoming more complex, cost of CRA on-boarding are also increasing & with advances in technology, it is bringing new opportunities and also challenges.

Key benefits to CRO, Pharmaceutical & Biotech Companies
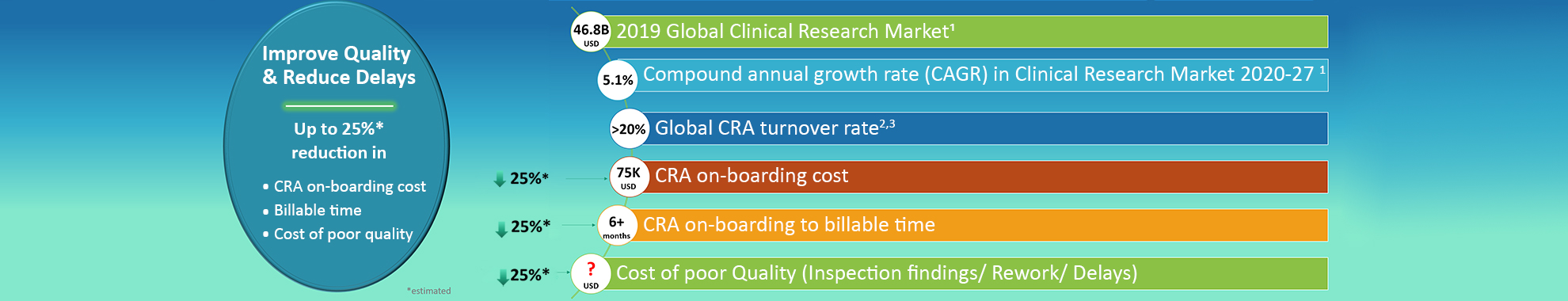
The global clinical trials market size was valued at USD 46.8 billion in 2019 and is expected to grow at a compound annual growth rate (CAGR) of 5.1% from 2020 to 2027. Globalisation of clinical trials, technological evolution, and demand for Contract Research Organisations (CROs) to conduct clinical trials are further projected to drive growth.
Most CROs are struggling with CRA retention; an ongoing challenge & not new to the industry and has experienced turnover at or above 20% for the past few years. The cost of on-boarding CRAs are also increasing & with advances in technology within clinical research (Risk Based Monitoring, Virtual trials); it is even more important for CRAs to gain a deeper, better and quicker understanding of new world of monitoring alongside of changing regulatory landscape. This has not only impact on cost associated with onboarding but also with time required to onboard CRA & cost associated with poor delivery of monitoring.
Using the big data available within public domain related to various regualtory rules, laws, guidelines & several thousands of non-compliances (inspection findings) GCP COMPASS; has converted all this data, using Artifical Intellience, Deep Learning techniques & GCP domain expertise, into a powerfull & interactive online learning tool. This tool has thousands of questions and each question is connected with GCP specific area, references in the form of flashcards & associated non-compliances, where available. This rich knowledge & powerful use of of technology will help user to connect theory to practice and then also understand the cosequences of non-compliance. We expect this intern will help up to 25% reduction in CRA on-boarding cost, billable time & cost of poor quality.
It is also important to note that the questionnaire in the tool will not be static and will ensure it is upto date with changing regulatory landscape and therefore you will always have something to learn and learn right things everytime and that is the commitment of GCP COMPASS.
1: https://www.grandviewresearch.com/industry-analysis/global-clinical-trials-market
2:https://www.outsourcing-pharma.com/Article/2019/01/03/CRO-industry-still-plagued-by-CRA-turnover-Report
3:https://www.businesswire.com/news/home/20200107005822/en/High-Levels-Turnover-Plague-CRO-Companies-Rates
