Why you need GCP COMPASS ?
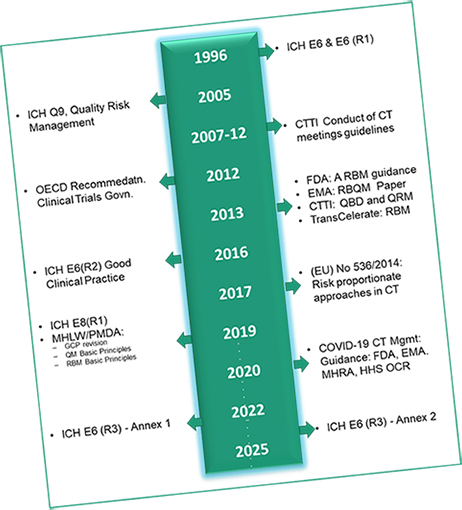
Since 1996 adoption of ICH E6 GCP, clinical trials have evolved substantially with increases in globalisation, study complexity, and technological capabilities. This also means; approach to Good Clinical Practice (GCP) needs modernisation to keep pace with the scale and complexity of clinical trials and to ensure appropriate use of technology.
While, we understand ICH E6 give sponsors flexibility to implement innovative approaches, this also has resulted in misinterpretation and sometime implementation in ways that keeps us away from innovation.
Therefore, we believe there is a need to facilitate broad and consistent international implementation of new methodologies through efforts from biopharmaceutical organisations in consultation with regulatory agencies. This is where, GCP COMPASS aims to bring practical knowledge, understand global requirements for conduct of a clinical trial and; provide a pragmatic approach to respect local needs/ challenges. It will also help organisations to navigate and deliver a reliable data.